Researchers Reveal the Oxygen Fugacity Increases in Exhumed Continental Slabs by Identifying Unusual Low-pressure Rutile GrowthUpdate time:05 08, 2018
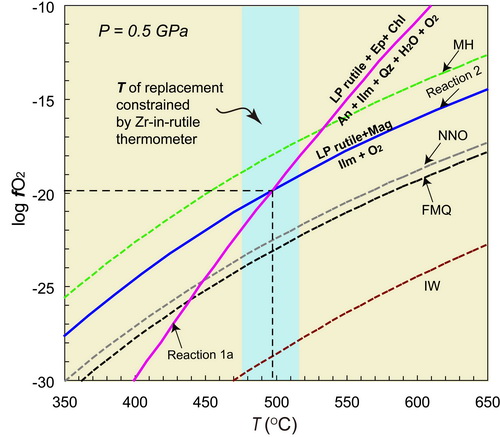
Fig. 1 Rutile (dark-colored mineral) in UHP eclogites (image by GUO Shun) Rutile (TiO2) is a common accessory mineral found in high-grade metamorphic rock (Fig. 1). This mineral has had wide geologic applications in the investigations of the formations and evolutions of orogenic belts. For example, it has been used to obtain the following information: (1) Temperature conditions (Zr-in-rutile thermometers); (2) Times (U-Pb dating); and (3) Chemical differentiations (Hf-O isotope and Nb-Ta ratio) during metamorphism. It is believed that understanding the growth mechanism and stability fields of rutile is the premise and key to both the rigorous geologic applications and reasonable interpretations in future geologic investigations. Both the available experimental and natural observations have indicated that rutile was mainly formed under relatively high-pressure (HP) or high-temperature conditions during the prograde metamorphic stages. However, Associate Professor GUO Shun and his colleagues from the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS), investigated an unusual case of retrograde rutile growth during cooling by replacing Fe-Ti oxides in retrograded eclogites from the Dabie ultra-HP (UHP) terrane. In their study, the detailed petrographic observations, mineral chemical analyses, and Zr-in-Rutile thermometry indicated that the rutile had formed under greenschist-facies conditions (approximately 0.5 GPa; 475 to 515°C), through a mechanism of interface-coupled dissolution-precipitation. The thermodynamic modeling indicated that the formation of the retrograde low-pressure (LP) rutile required relatively high oxygen fugacity (fO2) conditions, approximately 2.5 to 4.5 logfO2 units higher than the FMQ (fayalite-magnetite-quartz) reference buffer (Fig. 2). The in situ Sr isotopic analyses of the epidote suggested that the infiltrating fluid which was involved in the replacement reactions of the greenschist-facies were derived from the surrounding granitic gneisses. When compared with the eclogite-facies rutile, it was found that the LP rutile was characterized by lower Nb and Ta content and Nb/Ta ratios, and higher Cr and V content, which provided a geochemical fingerprint for distinguishing both types of rutile.
Fig. 2 Log fO2-T diagram showing the oxygen fugacity conditions of the LP rutile growth(image by GUO Shun) The results of this study indicated the following: (1) The rutile is able to grow during the retrograde LP metamorphic stages in a metabasite system; (2) Since various stages of ruitle potentially exist in high-grade metamorphic rock, it is important to distinguish each type of rutile before performing such geologic applications as thermometry, dating, and tracing; (3) The UHP eclogites in subducting continental slabs experience strong infiltrating of external high-fO2 fluids during exhumation; and (4) The occurrence of LP rutile can be used as an sensitive indicator of fO2 increases in subducting slabs. This study wassupported by the National Basic Research Program of China (2015CB856103); the National Science Foundation of China (41672059, 41372079); and the Youth Innovation Promotion Association CAS (2017090). The results of this study were published in the American Mineralogist(Guo et al.; The unusual replacement of Fe-Ti oxides by rutile during retrogression in amphibolite-hosted veins (Dabie UHP terrane): A mineralogical record of the fluid-induced oxidation processes in exhumed UHP slabs; American Mineralogist, 2017, 102: 2268 - 2283). This study was also selected as a highlights and breakthroughs article by the editor of the American Mineralogist, and was introduced by Dr. AM Cruz-Uribe from the University of Maine (Cruz-Uribe, Rutile: A novel recorder of high-fO2 fluids in subduction zones, 2017, 44(3):1302 - 1402).
|
Contact
GUO Shun
Phone: +86-10-82998534 The Institute of Geology and Geophysics, Chinese Academy of Sciences E-mail: guoshun@mail.iggcas.ac.cn Related Articles
Reference
|
-
SIMSSecondary Ion Mass Spectrometer Laboratory
-
MC-ICPMSMultiple-collector ICPMS Laboratory
-
EM & TEMElectron Microprobe and Transmission Electron Microscope Laboratory
-
SISolid Isotope Laboratory
-
StIStable Isotope Laboratory
-
RMPARock-Mineral Preparation and Analysis
-
AAH40Ar/39Ar & (U-Th)/He Laboratory
-
EMLElectron Microscopy Laboratory
-
USCLUranium Series Chronology Laboratory
-
SASeismic Array Laboratory
-
SEELaboratory of Space Environment Exploration Laboratory
-
PGPaleomagnetism and Geochronology Laboratory
-
BioMNSFrance-China Bio-mineralization and Nano-structure Laboratory

 Print
Print Close
Close