Researchers Find New Generation of Organic Sulfur Compounds and Diamondoids Under Deep Burial DolostonesUpdate time:09 18, 2016
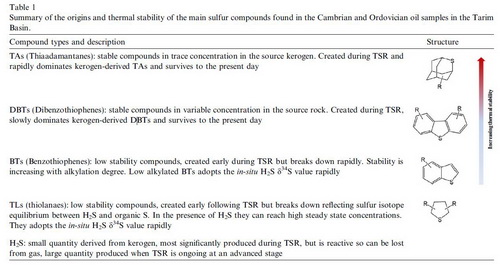
Deep burial reservoirs (4500-7000m) are a key target for China's petroleum exploration. Under deep burial or high temperature conditions, the stability of hydrocarbons is one of the main concerns. In fact, petroleum is expected not only to crack, but also to react with sulfates (through thermochemical sulfate reduction, TSR) to produce CaCO3(or CO2)and H2S: Hydrocarbons + Ca2++ MgSO4→CaCO3 + Mg2+ + H2S + Organic sulfur compounds Prof. CAI Chunfang has studies TSR for more than 20 years. He and his colleagues presented the first evidence that thiols and thiolanes can be generated from back-reactions of TSR-H2S with hydrocarbons. In a more recent study, they measured δ34S values of individual organic sulfur compounds from Tazhong oils from the Cambrian and Ordovician reservoirs, found that unstable thiolanes and benzothiophens were rapidly generated and remained stable when associated with TSR-H2S, and will keep in equilibration with H2S and its sulfur isotopic composition. Thus these compounds have δ34S values similar to newly generated or charged H2S. In contrast, thermally stable thiadiamondoids (TDs) and dibenzothiophenes (DBTs) were generated slowly during the early TSR stage, and more quickly with increasing degree of TSR. These compounds are stable under present reservoir conditions and have δ34S values representative of all sources. Oils in this area have < 20 mg/g TDs and up to 1300 mg/g DBTs derived from source rocks. Thus, during the early TSR, DBTs may be derived mainly from source rocks and their sulfur isotopic compositions can be used for oil-source rock correlation purpose. Conversely, TDs-δ34S values represent those of TSR-H2S and sulfates reactants (in general, there exists no significant sulfur isotope fractionation during natural TSR). Based on these, CAI and his team found that parameters such as individual sulfur compounds δ34S values and TDs concentrations can be used to determine migration and mixing of different oils from the Cambrian source rocks and reservoirs. This result shows that significant amounts of oils accumulated in the Cambrian and thus Cambrian reservoirs may have a good prospect for future petroleum exploration. TSR from deep petroleum sources generated not only thiolanes, DBTs and TDs, but also polythiadiamondoids including dithiaadamantanes, trithiaadamantanes and tetrathiaadamantanes. To the best of the authors'knowledge, tetrathiaamantanes are detected from oils in their study for the first time. TSR may have newly generated diamondoids and resulted in up to two orders of magnitude differences in 3- and 4-MD (or total diamondoids) concentration. This is because the differences cannot be explained simply by concentration as the result of cracking or oxidization of unstable compounds. TSR has enhanced isomerization of diamondoids, resulting in an increase in diamondoid–based maturity and source rock lithology parameters with increasing degree of TSR. Thus these parameters cannot be used as the proxies to reflect maturity in TSR active areas. Concentrations of H2S and especially thiadiomondoids are usually used to reflect the degree of TSR. The question remains, however, how to determine if TSR occurs or not in terms of petrology? Dr. LEI Jiang, Prof. CAI and other coauthors found that calcite precipitating from TSR environment shows a prominent positive Eu anomaly and an unusually high-chondritic Y/Ho ratio. Both yttrium versus holmium fractionation and Eu2+ oxidation to Eu3+ must have occurred during TSR. Hence, a positive Eu anomaly and an elevated Y/Ho ratio may be used as effective proxies to differentiate calcite resulting from TSR from ordinary calcite cement. This is especially useful when carbon isotope analysis cannot be used to give an unambiguous interpretation of calcite origin.
Fig. 1: Synthesis diagram representing the sulfur isotope values and thiadiamondoids concentrations of the main classes of organosulfur compounds in the four groups of oils discerned in this study.(Image by CAI et al.) Fig. 2 Mass chromatogram of m/z 236 C2–tetrathiaadamantanes of ZS1C condensate and their mass spectra. The spectra are not entirely those of the pure compounds.(Image by CAI et al.)
|
Contact
CAI Chunfang
Division: Petroleum Resources Goup: Sedimentology Phone: 86-10-82998127 E-mail: cai_cf@mail.iggcas.ac.cn Related Articles
Reference
|
-
SIMSSecondary Ion Mass Spectrometer Laboratory
-
MC-ICPMSMultiple-collector ICPMS Laboratory
-
EM & TEMElectron Microprobe and Transmission Electron Microscope Laboratory
-
SISolid Isotope Laboratory
-
StIStable Isotope Laboratory
-
RMPARock-Mineral Preparation and Analysis
-
AAH40Ar/39Ar & (U-Th)/He Laboratory
-
EMLElectron Microscopy Laboratory
-
USCLUranium Series Chronology Laboratory
-
SASeismic Array Laboratory
-
SEELaboratory of Space Environment Exploration Laboratory
-
PGPaleomagnetism and Geochronology Laboratory
-
BioMNSFrance-China Bio-mineralization and Nano-structure Laboratory

 Print
Print Close
Close