High-precision constant energy MD simulations show the formation of structure-I methane hydrate with a high degree of crystallinityUpdate time:05 18, 2015
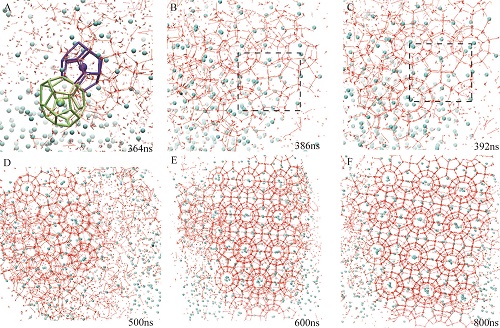
Gas hydrates are multi-component crystalline compounds made from space-filling cages of water molecules that accommodate small guest molecules such as methane, nitrogen, carbon dioxide, and hydrogen. Gas hydrates have received recent attention because of their scientific and industrial importance – for example, in energy recovery, climate science, energy storage, and seafloor stability. However, the formation mechanisms of gas hydrates are still not clearly understood. Recent work in the area of understanding hydrate nucleation mechanisms has postulated a mechanism by which hydrates nucleate through an amorphous intermediate which then anneals (or grows) to the crystal phase. However, computational investigations of hydrate nucleation to date have not reported conclusive evidence showing that the crystalline phase of structure-I can directly form so predominantly as to suppress the amorphous phase and span periodic boundaries. Ph. D. student Zhengcai Zhang and his supervisor Prof. Guang-Jun Guo, Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS), in cooperation with Dr. M. R. Walsh from Chevron Energy Technology Company, performed six 1 μs high-precision constant energy molecular dynamics (MD) simulations (ΔE<0.03%). While five of the simulations nucleate to the amorphous hydrate phase, one simulation nucleates more directly to a structure-I crystalline template (Fig. 1) of methane hydrate with long-range order spanning the simulation box across periodic boundaries (Fig. 2). This investigation reports the most compelling evidence to date that gas hydrates can nucleate not only to amorphous solids, but also to ordered solids with a high degree of crystallinity, and that the direct nucleation of the globally-stable hydrate phase is possible. This study has published in February 2015 in the RSC journal of Physical Chemistry Chemical Physics (Zhengcai Zhang, M. R. Walsh, and Guang-Jun Guo, Microcanonical molecular simulations of methane hydrate nucleation and growth: evidence that direct nucleation to sI hydrate is among the multiple nucleation pathways. Physical Chemistry Chemical Physics, 2015, 17: 8870-8876). Fig. 1 Snapshots of different stages for direct nucleation trajectory. Hydrogen bonds and water molecules are shown in red. Methane molecules are represented by cyan spheres. The green and blue cages are 51262 and 512 which connect with each other with a pentagon face, respectively. The dashed black square shows sI motifs at 386 ns and 392 ns.
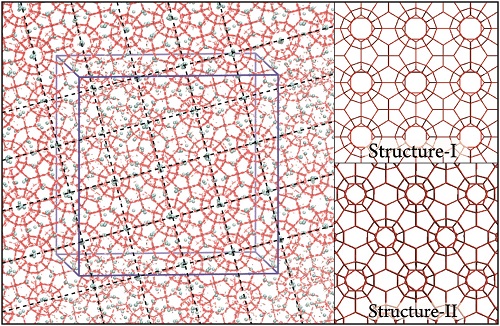
Fig. 2 Snapshot of the [001] crystallographic face from direct nucleation trajectory at 900 ns. Hydrogen bonds and water molecules are shown in red. Methane molecules are represented by cyan spheres. The blue box represents the system box. To show the long-range ordering, the periodic images in two directions are shown, both parallel to the page. The perpendicular or parallel nature of the dashed lines guides the eye in recognizing the sI template spanning the simulation box. The right panel shows the hydrogen-bonded network of perfect sI and sII hydrates.
|
Contact
Related Articles
Reference
|
-
SIMSSecondary Ion Mass Spectrometer Laboratory
-
MC-ICPMSMultiple-collector ICPMS Laboratory
-
EM & TEMElectron Microprobe and Transmission Electron Microscope Laboratory
-
SISolid Isotope Laboratory
-
StIStable Isotope Laboratory
-
RMPARock-Mineral Preparation and Analysis
-
AAH40Ar/39Ar & (U-Th)/He Laboratory
-
EMLElectron Microscopy Laboratory
-
USCLUranium Series Chronology Laboratory
-
SASeismic Array Laboratory
-
SEELaboratory of Space Environment Exploration Laboratory
-
PGPaleomagnetism and Geochronology Laboratory
-
BioMNSFrance-China Bio-mineralization and Nano-structure Laboratory

 Print
Print Close
Close