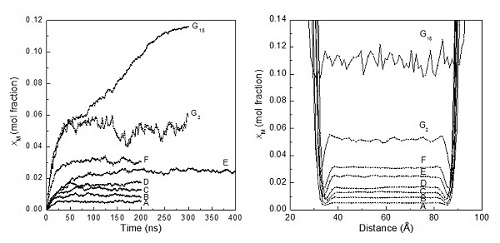
Solubility of aqueous methane under the metastable conditions: Implications for gas hydrate nucleationUpdate time:07 12, 2013
Professor GUO Guangjun and his team measure methane solubility under metastable conditions using molecular dynamics simulations to understand the prenucleation stage of methane hydrate formation. Three factors that influence solubility are considered: temperature, pressure, and the strength of the modeled van der Waals attraction between methane and water. Moreover, the naturally formed water cages and methane clusters in the methane solutions are analyzed. They find that both lowering the temperature and increasing the pressure increase methane solubility, but lowering the temperature is more effective than increasing the pressure in promoting hydrate nucleation because the former induces more water cages to form while the latter makes them less prevalent. With an increase in methane solubility, the chance of forming large methane clusters increases, with the distribution of cluster sizes being exponential. The critical solubility, beyond which the metastable solutions spontaneously form hydrate, is estimated to be ∼0.05 mole fraction in this work, corresponding to the concentration of 1.7 methane molecules/nm3. This value agrees well with the cage adsorption hypothesis of hydrate nucleation. Figure 1. Time evolution of xM(t) for all systems and profiles of xM(z) for all systems.(Image by GUO)
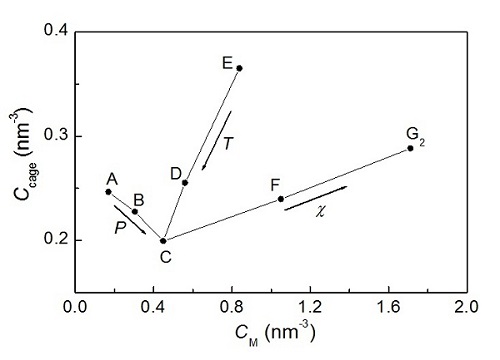
Figure 2. Relationship between methane concentration and cage concentration in solutions. (Image by GUO) Guo and Rodger. Solubility of aqueous methane under the metastable conditions: Implications for gas hydrate nucleation. J. Phys. Chem. B, 2013, 117:6498-6504 (Download Here)
|
Contact
Related Articles
Reference
|
-
SIMSSecondary Ion Mass Spectrometer Laboratory
-
MC-ICPMSMultiple-collector ICPMS Laboratory
-
EM & TEMElectron Microprobe and Transmission Electron Microscope Laboratory
-
SISolid Isotope Laboratory
-
StIStable Isotope Laboratory
-
RMPARock-Mineral Preparation and Analysis
-
AAH40Ar/39Ar & (U-Th)/He Laboratory
-
EMLElectron Microscopy Laboratory
-
USCLUranium Series Chronology Laboratory
-
SASeismic Array Laboratory
-
SEELaboratory of Space Environment Exploration Laboratory
-
PGPaleomagnetism and Geochronology Laboratory
-
BioMNSFrance-China Bio-mineralization and Nano-structure Laboratory

 Print
Print Close
Close