Effects of cage type and adsorption face on the cage-methane adsorption interaction: Implications for hydrate nucleation studiesUpdate time:07 09, 2013
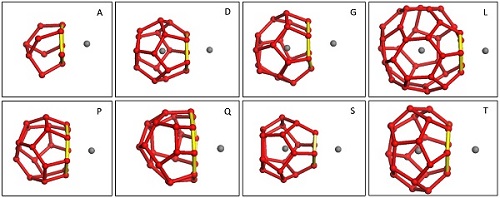
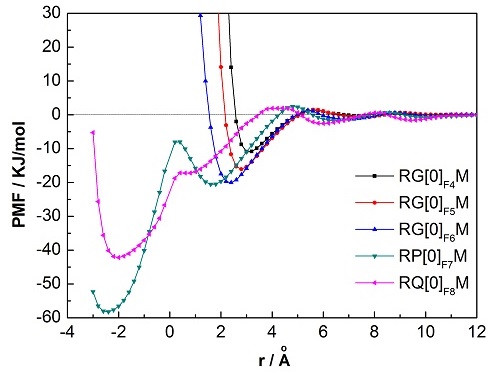
Ph.D. student LIU Chanjuan and her teacher GUO Guangjun investigate how the cage type and adsorption face affect the potential of mean force (PMF) for hydrate nucleation. They find that cage types have almost no effect on the interaction, except that the incomplete A cage shows a slightly weaker attraction to methane than do the other complete cages. However, the size of the adsorption face has a strong effect on the cage–methane adsorption. When the face size is increased from 4 to 6, the first well of the PMF shifts closer to the adsorption face and becomes deeper. This shows that a larger face attracts the dissolved methane more strongly, and thus implies that a preferential direction of cage development may exist during hydrate nucleation and growth. However, once the face is 7-membered or more, it can no longer adsorb methane and actually transforms into a hole that allows the methane to enter the cage. This finding provides a solid foundation for our recently established cage identification method (i.e., FSICA), and strengthens the idea that a water cluster can be distinguished as a cage because it is self-enclosed to its guests.
Figure 1. The different cages used in this work. (Image by LIU)
Figure 2. The cage–methane PMFs for the pentagonal adsorption face. (Image by LIU) Liu et al. Effects of cage type and adsorption face on the cage-methane adsorption interaction: Implications for hydrate nucleation studies. Chemical Physics Letters, 2013, 575: 54-58 (Download Here)
|
Contact
Related Articles
Reference
|
-
SIMSSecondary Ion Mass Spectrometer Laboratory
-
MC-ICPMSMultiple-collector ICPMS Laboratory
-
EM & TEMElectron Microprobe and Transmission Electron Microscope Laboratory
-
SISolid Isotope Laboratory
-
StIStable Isotope Laboratory
-
RMPARock-Mineral Preparation and Analysis
-
AAH40Ar/39Ar & (U-Th)/He Laboratory
-
EMLElectron Microscopy Laboratory
-
USCLUranium Series Chronology Laboratory
-
SASeismic Array Laboratory
-
SEELaboratory of Space Environment Exploration Laboratory
-
PGPaleomagnetism and Geochronology Laboratory
-
BioMNSFrance-China Bio-mineralization and Nano-structure Laboratory

 Print
Print Close
Close