Biosilicification, i.e., the formation of biological structures composed of silica, has wide distribution among eukaryotes. It plays a major role in global biogeochemical cycles and has driven the decline of dissolved silicon in the oceans throughout geological time.
A research team led by Profs. LI Jinhua and PAN Yongxin from the Institute of Geology and Geophysics of the Chinese Academy of Sciences (IGGCAS) has discovered a novel magnetotactic bacterium that forms intracellular, amorphous silica globules.
This finding reveals intracellularly controlled silicification within prokaryotes and suggests a previously unobserved influence on the biogeochemical Si cycle that was operational during early Earth history.
The study was published in Science Advances on May 13.
Silicon is the second most abundant element in Earth’s crust after oxygen. It is an essential element for life, both on land and in the ocean. The formation of biological structures composed of silica, known as biosilicification, is widely distributed in eukaryotes.
It has previously been proposed that the radiation of biosilicifying eukaryotes during the Phanerozoic drove secular decreases in dissolved silicon (DSi) in the global ocean, ultimately resulting in the low DSi concentrations seen today. However, strong evidence of such a process has remained elusive during the Precambrian.
Prof. LI Jinhua discovered one particular group of magnetotactic cocci in sediments collected from Lake Weiyanghu (WYH), located in Xi’an City, Shaanxi Province, China. This bacterium, tentatively named strain WYHC-5 and phylogenetically affiliated with the Nitrospirae phylum, belongs to a deep-branching group of magnetotactic bacteria (MTB).
"Electron microscopy analyses showed that strain WYHC-5 can accumulate a large amount of silicon within its cells in the form of amorphous silica globules, aside from forming magnetite-type magnetosomes and sulfur inclusions,” said Prof. LI.
The results of metagenomic analysis of the WYHC-5 genome have revealed the presence of a key gene possibly involved in silica deposition. Further assessment has revealed that the intracellular silica globules are also present in some other strains affiliated with the Proteobacteria phylum.
This study thus provides previously unrecognized structural and molecular evidence for intracellularly controlled biosilicification in prokaryotes. The results suggest a possible influence on the biogeochemical silicon cycle during early Earth history.
These findings also expand the record of silicifying prokaryotes from the genus Bacillus belonging to the Firmicutes phylum and the cyanobacterium Synechococcus to several MTB species affiliated with the Nitrospirae and the Proteobacteria phyla. However, the biosilicification pattern (i.e. the form and location of silicified products) is obviously different among bacterial groups.
The MTB strain WYHC-5 accumulates silicon intracellularly in the form of amorphous silica globules due to an estimated DSi concentration approximately 3,500-fold higher than that in surrounding water used for experiments. Therefore, it represents both a novel and a highly effective mode of prokaryotic biosilicification.
Phanerozoic cherts are primarily composed of silicified skeletons and thus are biological in origin. However, the origin of Precambrian cherts has long been an enigma, due to a lack of evidence that silica-secreting organisms were present in the Precambrian in sufficient abundance to have had a significant influence on the Si cycle. All MTB alive today are microaerobic and anaerobic, and versatile in their C, N, S, P and Fe metabolisms. They are also environmentally ubiquitous, living in various freshwater, brackish, marine, and hypersaline habitats, and even in hot springs.
"Taken together, these facts support the possibility that MTB lived in hypoxic environments in the Archaean Ocean at a point in early Earth history. Therefore, prokaryotes may play a role in driving the biogeochemical Si cycle in both modern environments and early Earth history, provided that prokaryotic biosilicification has an ancient origin rather than a recent horizontal gene transfer,” said Prof. PAN.
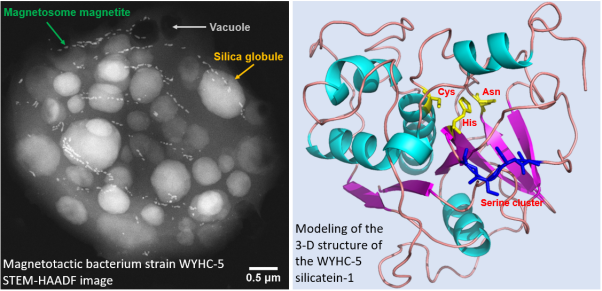
Fig. 1. Left panel: Scanning transmission electron microscopy image of one typical WYHC-5 cell acquired in the high angle annular dark field mode (STEM-HAADF) exhibiting multiple chains of magnetosome magnetite, numerous silica globules, and electron-transparent vacuoles. Right panel: Modeling of the 3-D structure of the WYHC-5 silicatein-1. The catalytic center of silicatein-1, comprising the amino acids Cys, His and Asn (yellow) and localization of the serine cluster (blue) are marked. Modified from Figs. 1 and 4 in Li et al., (2022 SA).
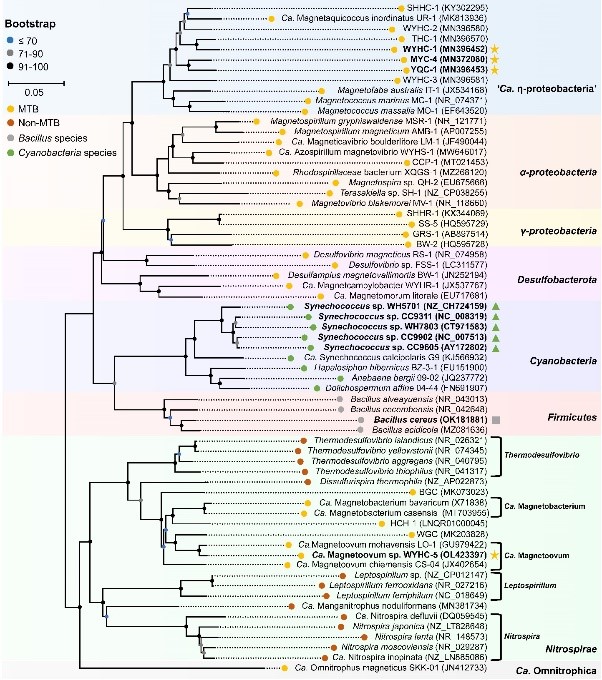
Fig. 2 Phylogenetic position of strain WYHC-5 and other silicifying bacteria. The silicifying bacteria are labeled in bold. Intracellular biomineralization of silica globules within the MTB system, formation of silica granules on the spore coat of Bacillus cereus, and silicification within cyanobacteria are respectively indicated with yellow stars, a gray square, and green triangles. From the Fig. 3 in Li et al., (2022 SA).
Contact:
LI Jinhua
Institute of Institute of Geology and Geophysics, Chinese Academy of Sciences
Phone:86-10-82998323
E-mail: lijinhua@mail.iggcas.ac.cn
PAN Yongxin
Institute of Institute of Geology and Geophysics, Chinese Academy of Sciences
Phone:86-10-8299406
E-mail: yxpan@mail.iggcas.ac.cn